Overview of the inflammatory response to neural infection by Toxoplasma gondii
- INTRODUCTION
Toxoplasma gondii (T. gondii) is an intracellular parasitic protozoan that is widely distributed across the globe, belonging to the phylum Apicomplexa. It is capable of persistently infecting any kind of warmblood animals, which can lead to death (David Sibley et al., 2009). The parasite has Felidae as its definitive host and other animals as an intermediate host. The main sour- ce of contamination is through contaminated water and undercooked meat, and placental transmission may also occur (Tenter et al., 2000). Thus, this zoonosis mainly affects the population with low purchasing power (Gar- cia Bahia-Oliveira et al., 2003), and co-infection with other endemic pathological agents was reported as a possible risk factor (Oliveira et al., 2020).
- T. gondii has a complex life cycle that provides a higher genetic variability, with the different genotypes framed in 3 clonal lines or variant There is a great divergence among virulence profiles, growth and migration from the strains (L. D. Sibley, 2009). The diversity that the parasite presents is decisive for its wide range of hosts, routes of transmission, and pathogenicity, making the parasite a great evolutionary success (Galal et al., 2019; Lorenzi et al., 2016).
In the parasite’s life cycle, there are different stage forms. The main forms that are covered here are responsible for the infection, proliferation, and persistence into the intermediate host. Tachyzoites presents high infectivity and rapid multiplication, being the main stage responsible for the complications and deaths that hosts present in the acute phase of the infection. On the other hand, bradyzoites is the slow multiplication and persistent stage contained in tissue cysts that are characteristics of the chronic phase of the infection. Both parasitic stages have the capacity to infect any nucleated cell in the host. (Dubey, 1998).
Thus, the host’s immune system exhibits a large variety of mechanisms that control the infection. The main mechanisms wrapped in this process are across the pro-inflammatory cytokine production and effector cellular immune response (Brasil et al., 2017). In this adverse environment, T. gondii protects itself from the combination of cell membranes that will later become the cystic wall of mature bradyzoite. From parasitic control under host cell protein pathways, the parasitophore vacuole is formed and within it the process of differentiating from tachyzoites to bradyzoites is initiated. (Dubey, 2008).
In all these processes, T. gondii also uses its surface proteins and secretory organelles (D. L. Sibley et al., 1999). The main proteins involved in parasite’s multiplication are those from family of surface antigens (SAG), microneome proteins (MIC), roptric proteins (ROP and RON) and granule proteins (GRA). The differentiation of T. gondii’s stage is accompanied by SRS (SAG-1 related sequence) superfamily. The cyst wall is also made up of se- veral proteins such as CST (cyst wall proteins) and MAG (matrix protein).
The tissue cysts can be found across all the body of the host during the chronic phase, meantime, they show a clear preference for muscle and nerve tissue. Thus, when the immune system not can control the infection of T. gondii, the central nervous system (CNS) become the inflammatory focus this being a widely studied subject (Schlüter & Barragan, 2019). Thereby, Toxoplasmic encephalitis is the main clinical manifestation of the infection in people with acquired immunodeficiency syndrome – AIDS (Vidal, 2019). In addition to classic clinical pictures, the presence of the parasite in the CNS is also widely associated with behavioral changes in its hosts (Fabiani et al., 2015). It is appointed that the cause of behavioral changes as an effect of the interaction of immune (Wang et al., 2019), hormonal (Tan & Vyas, 2016) and genetic processes (Bay-Richter et al., 2019) with the infection by T. gondii.
2 Methodology
Four articles recently published between the 2020 – 2019 period, were selected in the PubMed report in the NCBI database following “neuroinflammation” and “Toxoplasma gondii” as keyword research (Tab.1). The purpose of this review was to present an overview of the inflammatory mechanisms caused by T. gondii infection in a neural environment. Thus, articles were recommended that presen- ted different immunological perspectives on the infection interference in the CNS, finally drawing a gradual line of information that talk to each other.
3 Results
3.1 Humoral immune response to cystic wall antigens
A recent study realized with sera of 404 goats, 88 sheep, and 92 humans se- arched to compare the humoral response against protein agents typical of acute (SAG1 and GRA7) and chronic (CST 1 and SRS9) infection by T. gondii. The proposed study was to analyze the humoral immune response against bradyzoite and associated cyst wall antigens. The authors justify that T. gondii infection has been well reported to present a strong humoral response against tachyzoite antigens, however, the humoral immune response against typical agents of the chronic phase is poorly characterized (Deshmukh et al., 2020). Using ELISA, the authors tested antibodies derived from natural infection present in the serum of almost 50% of the samples, as expected, most of the sera that recognized the cyst wall antigen were T. gondii IgM- IgG+ whereas tachyzoite antigens was recognized by Tgondii IgM+ IgG- (Table 3 from the original article).
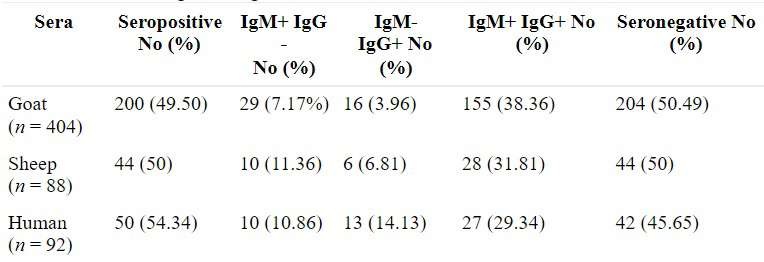
Note: Reprinted from “Toxoplasma gondii induces robust hu- moral immune response against cyst wall antigens in chronically infected animals and humans”, by Deshmukh et al., 2020.
The sera seropositive were tested for immunoreactivity of anti- gens characteristic of the cystic wall and the surface antigen of brady- zoites, CST1 and SRS9 respectively, and compared them with the surface antigen of tachyzoites SAG1 and with the antigen associated with the parasitophorous vacuole GRA7. Natural antibodies were also tested against the protein lysate produced by the authors, containing tachyzoites from representatives of the 3 clonal lines: RH (type I), ME49 (type II), and VEG (type III).
There is a robust production of the humoral immune response against cystic wall antigens in animals and humans infected with T. gondii (Fig. 1A), compared to the response presented from tachyzoite proteins. Similarly, is not possible to say about antigens from bradyzoites which present less hu- moral response than those cystic wall antigens. The authors argue that this difference may be due to the cysts wall being more exposed to immune cells than bradyzoites during rupture of the parasitized cell. All the results were found and validated by ELISA, Western blot and IFA analyzes.
3.2. Recruitment of monocytes to specific sites from Toxoplasma gondii infection in the CNS
Monocytes are immune cells commonly divided into 2 groups, inflammatory monocytes that are known to be the progenitors of macrophages and dendritic cells, and the “patrolling” monocytes that comprise in checking endothelial cell homeostasis. This division is mainly due to its characteristic functions and the profile of receivers coupled to its surface. During inflammatory events in mice, monocytes are recruited from the chemokine CCL2 that signals them by binding to the CCR2 receptors present on their surface. Involved in various immunological processes, they are highly found in the CNS during infection by T. gondii. Howe- ver, little is known about how, where, and when these cells enter during infection (Mitchell et al., 2014).
Therefore, Schneider et al. (2019) Carry out a study on the recruitment dynamics of inflammatory and patrolling monocytes in the blood and the CNS, analyzing the cerebral location of these monocytes in the animal model infected by the parasite Toxoplasma gondii.
Firstly, tracking of the frequency of immune cells in the blood of these animals was promoted on the fourth and sixth day postinfection (dpi). It is possible to identify a considered increase in inflammatory and patrolling monocytes at 6 dpi. An increase in CCL2 chemokine and cytokines typical of the infection profile in the animals’ serum was also observed, a result that contributes to the increase in monocytes. The recruitment of monocytes to the CNS was also evaluated, with infiltration being detected in the first week postinfection.
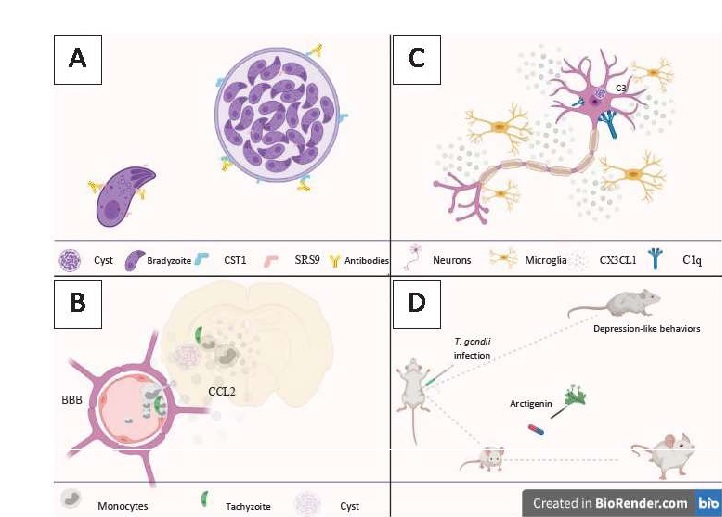
Considering 28 dpi as a chronic phase, the authors observed that there is an increase in monocytes in the CNS between 10 to 15 dpi, with the value being kept constant at 38 dpi. By longitudinal analysis in mice using “2-photon brain intravital imaging” through cranial Windows, it was shown that inflammatory monocytes are recruited to the blood-brain barrier within two weeks after infection. Reporting different behavior from rolling and crawling and free-flow movements, inflammatory monocytes accumulate in the lumen of the blood-brain barrier before transmigration to the parenchyma (Fig. 1B).
The authors were also able to significantly correlate monocytes at sites with clusters of T. gondii. When comparing infected animals with animals injected with LPS, it was elaborated that the monocytes infiltrated in animals infected with Toxoplasma gondii were mostly concentrated in the olfactory tubercle, whereas in animals injected with LPS, a diffuse distribution was found between several regions of the Brain. There is a consistent pattern of monocyte infiltration during an infection that coincides with the location and the detection period of the parasite in the brain. It can be understood that the location of these cells in the CNS is highly regionalized during an infection.
3.3. The persistence of Toxoplasma gondii infection promotes neurodegeneration associated with activation of microglia and the complement system
As seen earlier, infection by T. gondii remains from the cystic load that is distributed throughout the host’s body. With a clear preference for long-lived cells, neurons are the main nerve cells carrying cysts in the CNS. As a result, they are more exposed to possible consequences derived from the presence of the parasite. Thus, this article presents data on neurodegeneration and the immunological mechanisms involved therein (Li et al., 2019).
For this study, the authors infected CD-1 outbred mice with strain GT1, belonging to type I clonal lineage. Subsequently, the mice were divided into 5 groups based on their serological profile: (I) an unexposed control group; (II) an IgG-positive group with a high antigen matrix 1 (MAG1) positive (IgG + / MAG1 + high); (III) an IgG + / MAG1 + group low; (IV) a group exposed to Toxoplasma that did not develop MAG1 antibody (IgG + / MAG1-); and (V) a group exposed to Toxoplasma that did not develop an antibody response (IgG- / MAG1-). IgG being a positive indicator of parasite exposure, while MAG1 serves as a serological marker for cyst load.
The authors used Fluoro-JadeB (FJB), a well-known marker of degenerating neurons, to examine the brains of mice infected by the parasite. A persistent infection has been shown to promote cortical neurodegeneration that affects glutaminergic and gabaergic neurons. FJB-positive cells showed complement C1q and C3deposited on their surfaces and high expression of CX3CL1. As a result, activated microglia accumulate at the site of degeneration, surrounding the degenerative neurons (Fig. 1C). Interestingly, among the 5 groups, only mice that showed high MAG1 showed strong staining for FJB, with positive cells located predominantly in the cortex somatomotor (SC) and the anterior cingulate cortex (ACC).
Thus, the authors show that Cortical neurons in mice infected with Toxoplasma gondii demonstrate signs of degeneration. That is characterized by the deposition of components of the complement system and the overexpression of CX3CL1, a chemokine characteristic of an inflammatory neural environment responsible for binding to the CX3CR1 surface receptor of microglia that are known to mediate the phagocytic clearance of degenerating neurons. Interestingly, a positive relationship was found for greater expression of C1q and C3 in groups that showed high expression of MAG1, with the parasitic load being identified as a determinant for neurodegeneration.
3.4. Use of anti-inflammatory to inhibit depression-like behaviors in Toxoplasma gondii hosts
Chronic Toxoplasma gondii infection is widely associated with behavioral changes, neuroinflammation caused by the presence of the parasite in the brain is the main cause (Boillat et al., 2020). Certainly, the vast majority of articles that ad- dress this issue are limited to the promotion of infection from cystogenic strains, with the effect of behavioral change being mainly observed in models of infection from type II strains.
Cheng et al. (2020) used Arctigenin (AG), a bioactive that in previous isolated studies demonstrated anti-inflammatory, anti-T.gondii, and anti-depressant effects, to evaluate in vivo model and in vitro to explore its effects against de- pressive behaviors induced by Toxoplasma gondii infection and also its underlying molecular mechanisms.
The infectious strain used in the study was the type I representative, RH. This being a highly virulent strain, the first thing evaluated was the effects of AG on the survival of mice infected by the parasite. Infected mice treated with 100 mg/ kg of AG demonstrated similar survival to infected mice treated with sulfadiazine, common drug in the treatment of the disease (Bonfioli & Orefice, 2005), being chosen for further study (Fig. 1D). Thus, the experimental models were divided into 4 groups: (I) infected and untreated, (II) treated with 100 mg/kg of AG, (III) treated with sulfadiazine, and (IV) not infected or treated.
As a result of the in vivo study, it was demonstrated that the number of tachyzoites in the peritoneum cavity and SAG-1 expression in the brain decreased in the group treated with AG and Sulfadiazine when compared to the other infected groups. The clinical manifestations derived from the infection were observed from the sixth day onwards, being less observed in the group treated by AG than by sulfadiazine. Thus, after 6 dpi, mice from each group were separately subjected to behavioral depression tests such as tail suspension test (TST), forced swim test (FST), or sucrose preference test (SPT). Treatment with AG has been reported to be effective against de- pression in animals infected with the parasite. Analyzes of post-mortem neural injury showed that treatment with AG or sulfadiazine increased neural viability.
In in vitro studies, it was found that AG has a protective effect on microglia infected with Toxoplasma gondii, as it inhibits the intracellular proliferation of the parasite. The authors used Iba-1 as a marker of microglia activation, finding that in the infected groups there is an increase in the expression of this marker when compared to the non-infected ones, which indicates activation of the microglia during infection by the parasite. However, the groups treated with AG and sulfadiazine showed a decrease in the expression of Iba-1 to the untreated infected.
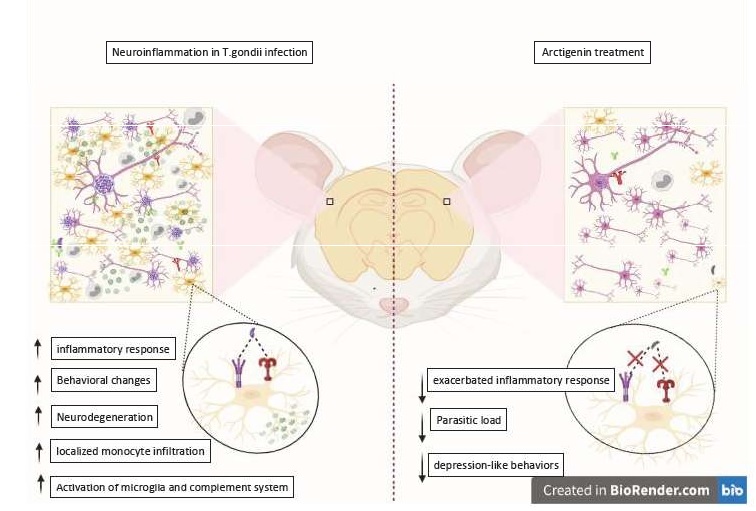
Investigating the effect of AG on the inflammatory response derived from acute infection in vitro, it was observed that the expression of levels of pro-inflammatory enzymes and cytokines is shown to be high in the untreated infected group compared to the uninfected group. While in the groups treated with AG or Sulfadiazine, the exacerbated inflammatory response was reduced.
Other effects of in vitro treatment observed were: Reduced levels of IDO (an enzyme that catalyzes the amino acid tryptophan) and monoamines and their effects, when compared to the results of untreated infected animals; Reversal of signaling via TLR4 / TRIF / NF-KB and TNFR1 / NF-kB via which promotes microglia activation (Fig.2). This effect was also observed in vivo.
3.5. Panorama of the neuroinflammatory environment from Toxoplasma gondii
infection and its effects
When we analyzed the 4 articles to synthesize part of the components that act in the inflammation, caused by the infection of the parasite in the CNS, it is possible to trace a neuroinflammatory environment typical of the infection by T. gondii (figure 2). Deshmukh et al., 2020 at first may seem lost in the central subject of this re- view, however, its contribution to the theme is extremely important. The authors show that both cysts and bradyzoites exhibit immunoreactivity to antibodies produced by different animals naturally infected by the parasite. This data is relevant because bradyzoites and cysts are almost completely responsible for the parasitic presence of T. gondii in the CNS. Previous articles indicate that antibodies play an important role in the death of neurons by T cells. Since the neural infiltration of immune cells is present in the infection, future studies must elucidate the role of antibodies in the neurodegeneration caused by the parasite.
Staying on the subject, Li et al. (2019) presents the neurons as the main cells affected by neuroinflammation caused by the parasite. demonstrating that glutaminergic and GABAergic neurons are in direct interaction with complement system components and microglia in an environment with CX3CL1 overexpression. This suggests an active process of neurodegeneration in specific regions of the brain. These results contribute to Schneider et al., 2019, which presents regions with the high parasitic load as the destination of monocytes infiltrating the CNS. Several articles demonstrate that the parasitic load, together with the high inflammatory response, is directly related to the behavioral changes that inter- mediate hosts present. With the olfactory tubercle being the main region with a positive relationship between parasites and infiltrating monocytes, the authors point to the existing connection between the olfactory tubercle and the olfactory bulb. It has been suggested that cell infiltration may play a fundamental role in the characteristic loss of aversion to predators, presented in animals infected with the parasite.
Corroborating the role of the inflammatory response in behavioral changes, Cheng et al 2020 demonstrate that the use of anti-inflammatory and anti-parasitic drugs reduces depression-like behavior in animals infected with virulent strains of Toxoplasma gondii. Here it is important to point out that the used strain belongs to a highly virulent clonal strain that usually kills the murine model in a few days, not commonly used in behavioral testing. However, studies show that part of the behavioral changes in humans occur from infection with type I strains.
A recent study also demonstrated a decrease in behavioral changes from anti-inflammatory treatment. The authors from this work used a strain belonging to type II linage and found a positive correlation between cysts loading, neuroinflammation, and hyperactivity. However, there were divergent results when using another animal model (Martynowicz et al., 2019).
In this case of this present study, interesting results were found using un- conventional strain. AG was able to attenuate the activation of microglia (which plays an important role in neurodegeneration as we saw earlier) and regulates the expression of neurotransmitters such as dopamine and serotonin from the interactions with specific pathways.
- Conclusion
In this review, from the analysis of 4 recent articles, which focus on impor- tant components for the neuroinflammatory environment. The neural inflammatory condition derived from infection by Toxoplasma gondii shows overexpression of chemokines that recruited resident and infiltrating immune cells to the focus of the infection. As Neurons being the main carriers of the parasite in the brain, other studies also demonstrate that the parasitic load is concentrated in specific locations of the CNS, mainly in the region of the cerebral cortex and olfactory bulb. In behavioral studies it is observed that the region in which the infection is concentrated is essential for the behavioral changes that the host will present. Thus, these articles presented here corroborate the infection literature. Adding new information on the underlying inflammatory mechanisms acting in the neural environment in the presence of T. gondii.
5. Appendices
- Abbreviations
ACC – Anterior cingulate cortex (ACC) AG – Arctigenin
AIDS – Acquired immunodeficiency syndrome
C1q – Complement C1q
C3 – Complement C3
CCL2 – C-C Motif Chemokine Ligand 2
CCR2 – C-C Motif Chemokine Receptor 2)
CD-1 – T-cell surface glycoprotein
CNS – Central nervous system
CST – Cyst wall proteins
CX3CL1 – C-X3-C motif chemokine ligand 1
CX3CR1 – C-X3-C Motif Chemokine Receptor 1
DPI – Days postinfection
FJB – Fluoro-JadeB
FST – Forced swim test
GFP – Green fluorescent protein
GRA – Dense granule protein
Iba-1 – Ionized calcium-binding adapter molecule 1
IDO – Indoleamine 2,3-dioxygenase
IFA – Immunofluorescence assay
IgG – Immunoglobulin G
IgM – Immunoglobulin M
LPS – Lipopolysaccharides
MAG – Matrix protein
MIC – Microneome proteins
NF-KB – Nuclear factor kappa B
RON – Rhoptry neck protein
ROP – Rhoptry protein
SAG –Surface Antigen
SC – Somatomotor cortex
SPT – Sucrose preference test
SRS – Bradyzoite surface antigen
- T. gondii – Toxoplasma gondii
TLR – Toll-like receptor
TNFR – Tumor necrosis factor receptor
TRIF – TIR-domain-containing adapter-inducing interferon-β
TST – Tail suspension test
7 . Acknowledgements
I would like to thank Humberto who encouraged me to write this article. My advisor Alba who lovingly helped me and was patient with me during the writing of this review. To my LBR colleagues who always inspire me. And to my friends Milena, Leticia, Layla, Cinthia and Amanda who were with me at all times. I would like to especially thank the Scientific English Writing Workshop and Conhecendo a Ciência Journal’s that provided me with this opportunity. I am also grateful to the Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF), to the La- boratório de Biologia de Reconhecer (LBR), to the Scientific Initiation Scholarship Program (PIBIC), and to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This work was supported by PIBIC-UENF.
References
Bay-Richter, C., Petersen, E., Liebenberg, N., Elfving, B., & Wegener, G. (2019). Latent toxoplasmosis aggravates anxiety- and depressive-like behaviour and suggest a role of gene-environment interactions in the behavioural response to the parasite. Behavioural Brain Research, 364(February), 133–139. https://doi.org/10.1016/j.bbr.2019.02.018
Boillat, M., Hammoudi, P. M., Dogga, S. K., Pagès, S., Goubran, M., Rodriguez, I., & Soldati-Favre, D. (2020). Neuroinflammation-Associated Aspecific Manipulation of Mouse Predator Fear by Toxoplasma gondii. Cell Reports, 30(2), 320-334.e6. https://doi.org/10.1016/j.celrep.2019.12.019
Bonfioli, A. A., & Orefice, F. (2005). Toxoplasmosis. Seminars in Ophthalmology, 20(3), 129–141. https://doi. org/10.1080/08820530500231961
Brasil, T. R., Freire-de-Lima, C. G., Morrot, A., & Arnholdt, A. C. V. (2017). Host-Toxoplasma gondii coadaptation leads to fine tuning of the immune Response. Frontiers in Immunology, 8(SEP), 1–9. https://doi.org/10.3389/fimmu.2017.01080
Cheng, J. H., Xu, X., Li, Y. B., Zhao, X. D., Aosai, F., Shi, S. Y., Jin, C. H., Piao, J. S., Ma, J., Piao, H. N., Jin, X. J., & Piao, L. X. (2020). Arctigenin ameliorates depression-like behaviors in Toxoplasma gondii-infected intermediate hosts via the TLR4/NF-κB and TNFR1/NF-κB signaling pathways. International Immunopharmacology, 82(October 2019), 106302. https://doi.org/10.1016/j. intimp.2020.106302
David Sibley, L., Khan, A., Ajioka, J. W., & Rosenthal, B. M. (2009). Genetic diversity of Toxoplasma gondii in animals and hu- mans. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1530), 2749–2761. https://doi.org/10.1098/ rstb.2009.0087
Deshmukh, A. S., Gurupwar, R., Mitra, P., Aswale, K., Shinde, S., & Chaudhari, S. (2020). Toxoplasma gondii induces robust humoral immune response against cyst wall antigens in chronically infected animals and humans. Microbial Pathogenesis, 152(November 2020), 104643. https://doi.org/10.1016/j.micpath.2020.104643
Dubey, J. P. (1998). Advances in the life cycle of Toxoplasma gondii. International Journal for Parasitology, 28(3), 1019–1024. https://www.ncbi.nlm.nih.gov/pubmed/?term=Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol.1998;28:1019–24.
Dubey, J. P. (2008). The history of Toxoplasma gondii – The first 100 years. Journal of Eukaryotic Microbiology, 55(6), 467–475. https://doi.org/10.1111/j.1550-7408.2008.00345.x
Fabiani, S., Pinto, B., Bonuccelli, U., & Bruschi, F. (2015). Neurobiological studies on the relationship between toxoplasmosis and neuropsychiatric diseases. Journal of the Neurological Sciences, 351(1–2), 3–8. https://doi.org/10.1016/j.jns.2015.02.028
Galal, L., Hamidović, A., Dardé, M. L., & Mercier, M. (2019). Diversity of Toxoplasma gondii strains at the global level and its determinants. Food and Waterborne Parasitology, 15. https://doi.org/10.1016/j.fawpar.2019.e00052
Garcia Bahia-Oliveira, L. M., Jones, J. L., Azevedo-Silva, J., Alves, C. C. F., Oréfice, F., & Addiss, D. G. (2003). Highly endemic, water- borne toxoplasmosis in North Rio de Janeiro State, Brazil. Emerging Infectious Diseases, 9(1), 55–62. https://doi.org/10.3201/ eid0901.020160
Li, Y., Severance, E. G., Viscidi, R. P., Yolken, R. H., & Xiao, J. (2019). Persistent toxoplasma infection of the brain induced neu- rodegeneration associated with activation of complement and microglia. Infection and Immunity, 87(8), 1–12. https://doi. org/10.1128/IAI.00139-19
Lorenzi, H., Khan, A., Behnke, M. S., Namasivayam, S., Swapna, L. S., Hadjithomas, M., Karamycheva, S., Pinney, D., Brunk, B. P., Ajioka, J. W., Ajzenberg, D., Boothroyd, J. C., Boyle, J. P., Dardé, M. L., Diaz-Miranda, M. A., Dubey, J. P., Fritz, H. M., Gennari, S. M., Gregory, B. D., … Sibley, L. D. (2016). Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nature Communications, 7. https://doi.org/10.1038/ncomms10147
Martynowicz, J., Augusto, L., Wek, R. C., Boehm, S. L., & Sullivan, W. J. (2019). Guanabenz Reverses a Key Behavioral Chan- ge Caused by Latent Toxoplasmosis in Mice by Reducing Neuroinflammation. MBio, 10(2), 1–15. https://doi.org/10.1128/ mBio.00381-19
Mitchell, A. J., Roediger, B., & Weninger, W. (2014). Monocyte homeostasis and the plasticity of inflammatory monocytes. Cellular Immunology, 291(1–2), 22–31. https://doi.org/10.1016/j.cellimm.2014.05.010
Oliveira, L. R. P., Martins, L. M., Souza, R. D. C., Scheidegger De Castro, Y., Nascimento, L. S., Da Silva, J. A., Nahn Junior, E. P., Da Silva, W. D., & Peixoto-Rangel, A. L. (2020). Serological evidence of Toxoplasma gondii infection as potential risk for the develo- pment of lepromatous leprosy in an endemic area for both neglected tropical diseases in Brazil. Infectious Diseases of Poverty, 9(1), 1–10. https://doi.org/10.1186/s40249-020-0636-3
Schlüter, D., & Barragan, A. (2019). Advances and challenges in understanding cerebral toxoplasmosis. Frontiers in Immunology, 10(FEB), 1–13. https://doi.org/10.3389/fimmu.2019.00242
Schneider, C. A., Figueroa Velez, D. X., Azevedo, R., Hoover, E. M., Tran, C. J., Lo, C., Vadpey, O., Gandhi, S. P., & Lodoen, M. B. (2019). Imaging the dynamic recruitment of monocytes to the blood–brain barrier and specific brain regions during Toxoplasma gondii infection. Proceedings of the National Academy of Sciences of the United States of America, 116(49), 24796–24807. https://doi.org/10.1073/pnas.1915778116 Sibley, D. L., Mordue, D., & Howe, D. K. (1999). Experimental approaches to understanding virulence in taxoplasmosis. Immunobiology, 201(2), 210–224. https://doi.org/10.1016/S0171-2985(99)80061-8
Sibley, L. D. (2009). Development of forward genetics in Toxoplasma gondii. International Journal for Parasitology, 39(8), 915-924. https://doi.org/10.1016/j.ijpar2009.02.011
Tan, D., & Vyas, A. (2016). Toxoplasma gondii infection and testosterone congruently increase tolerance of male rats for risk of reward forfeiture. Hormones and Behavior, 79, 37–44. https://doi.org/10.1016/j.yhbeh.2016.01.003
Tenter, A. M., Heckeroth, A. R., & Weiss, L. M. (2000). Toxoplasma gondii: From animals to humans. International Journal for Parasitology, 30(12–13), 1217–1258. https://doi.org/10.1016/S0020-7519(00)00124-7
Vidal, J. E. (2019). HIV-Related Cerebral Toxoplasmosis Revisited: Current Concepts and Controversies of an Old Disease. Journal of the International Association of Providers of AIDS Care, 18, 1–20. https://doi.org/10.1177/2325958219867315
Wang, T., Sun, X., Qin, W., Zhang, X., Wu, L., Li, Y., Zhou, C., Zhou, H., He, S., & Cong, H. (2019). From inflammatory reactions to neurotransmitter changes: Implications for understanding the neurobehavioral changes in mice chronically infected with Toxoplasma gondii. Behavioural Brain Research, 359(September 2018), 737–748. https://doi.org/10.1016/j.bbr.2018.09.011
This article has been developed at the 1st Scientific English workshop, a partnership between the Pro-Rectory of Postgraduate and Research (ProPPG-UENF), the International and Institutional Affairs Advisory (ASSAII-UENF), and the Program of Scientific and Technological Initiation (PIBi).
Title: Overview of the inflammatory response to neural infection by Toxoplasma gondii
Type of publication: Literature review (short)
Name: Milena dos Santos Tavares da Silva
Publishing name: SILVA, MST
Field of Study: Immunology
Institution and Term: Universidade Estadual do Norte Fluminense Darcy Ribeiro (UENF)

About the author
I am a 2021 undergraduate in Biological Sciences at UENF. Since my first year I have been working at the Laboratory of Recognition Bio- logy (LBR), where I am developing my Scientific Initiation (IC), which aims to evaluate the behavior of mice infected with atypical strains of the parasite Toxoplasma gondii, found in Campos dos Goytacazes and surroundings, under the guidance of Professor Dr. Alba Lucínia Peixoto Rangel. In parallel, I am the student representa- tive of the collegiate of Biological Sciences and I am an active member of the Academic Directory of Biology Paubrasilia echinata.
Contact milena.santos.tavares@gmail.com

